Cilvēka organismam novecojot, tiek traucēta adekvāta to vielu izvadīšana, kuru uzkrāšana veicina slimību attīstību.
Jau sen daudzu saslimšanu ārstēšanai lietoja asins nolaišanu. Vēl Hipokrāts rakstīja, ka “medicīna – tā ir atņemšana un pielikšana. Atņemšana tā, kas lieks, un pielikšana tā, kas trūkst. Un tas, kas to dara labāk, ir labākais ārsts”.
Rīgas 1.slimnīcas Aferēzes centrs piedāvā mūsdienīgas ārstnieciskas asins attīrīšanas metodes
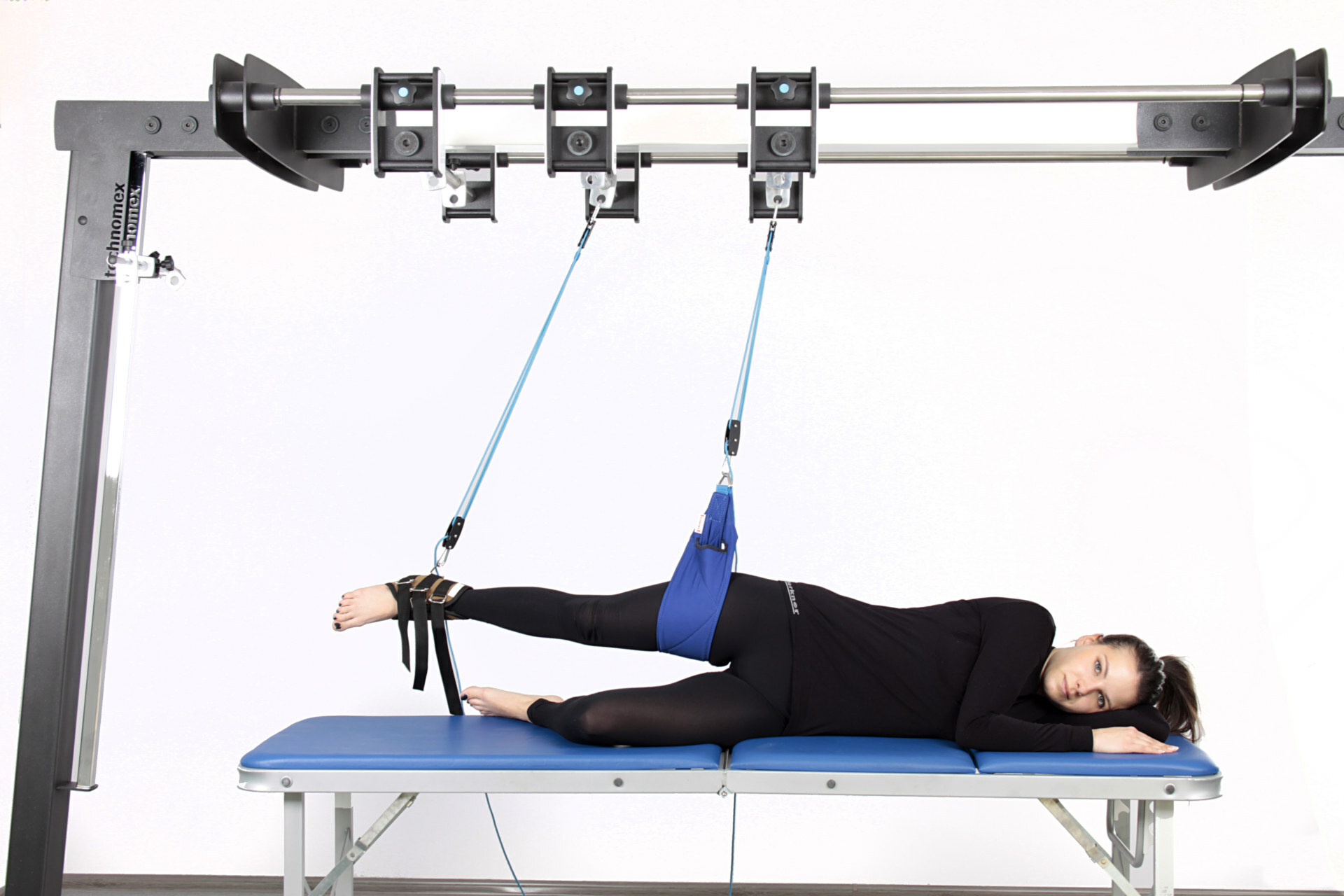
Plazmaferēze ir nesāpīga, droša, un moderna asins attīrīšanas metode, kas balstīta uz asins sadalīšanu ar speciālās aparatūras palīdzību. Notiek asins šķidrās daļas – plazmas, kurā atrodas šlakvielas un dažādu slimību uzturošas substances, noņemšana. Noņemtais plazmas tilpums tiek kompensēts ar aizvietojošiem šķīdumiem. Organisma asins šūnas tiek saglabātas. Sākumā šlakvielas izvadās no asinīm, bet pēc tam no audiem, kas ļauj panākt pamatīgu organisma attīrīšanu. Plazmas vietā ievadītie šķidrumi atšķaida asinis, mazina viskozitāti un uzlabo mikrocirkulāciju.
Plazmafērēze ir procedūra
kuras laikā plazma (asins šķidra daļa) tiek atdalīta no asins šūnu elementiem: eritrocītiem, leikocītiem, trombocītiem un citām šūnām ekstrakorporāli. Rezultātā, nepieciešamā daļa tiek noņemta, bet pārējā atgriezta pacientam.
Plazmaferēzes procedūras tiek veiktas gan ar manuālo, gan ar automātisko metodi, savukārt citaferēzes – ar automātisko metodi.
Termini plazmaferēze (PA) un plazmas apmaiņa apzīmē dažādas procedūras. Plazmaferēze attiecas uz procedūru, kurā plazma ir atdalīta no asinīm vai nu ar centrifugēšanu vai membrānu filtrāciju. Pēc tam, kad atdalītas plazma, ar to var manipulēt dažādos veidos.
Plazmas apmaiņa nozīmē, ka plazma pilnīgi tiek aizstāta ar rezerves šķidrumu.
Atkarībā no saslimšanas, plazmaferēze var būt izmantota kā pamatārstēšanas metode un papildārstēšanas metode.
Indikācijas neatliekamai pamatārstēšanas plazmaferēzei:
myasthenia gravis krīze,
DIK sindroms,
hemolitīsks urēmisks sindroms,
Crush-sindroms,
akūtas alerģiskas reakcijas – Kvinkes tūska,
eksotoksikozes – intoksikācijas (medikamentu, sēnēm, ķīmiskām vielām utt.),
endotoksikozes – sepse, hiperbilirubinēmija, abdeguma slimība),
trombotiskā trombocitopēniskā purpura un citi.
Indikācijas plānotai plazmaferēzei:
autoimūnās saslimšanas, krioglobulinēmija,
hiperviskozitāte pie mielomas slimības,
valdenstrēma makroglobulinēmija,
pemphigus vulgaris,
potfirija un citi.
Citaferēzes procedūras laikā
tiek atdalīti un eliminēti leikocīti (leikocitoferēze), trombocīti (trombocitoferēze) vai eritrocīti (eritrocitoferēze) onkohematoloģisku slimību un citos gadījumos.
Plazmaferēzi pielieto
alergoloģijā (jebkura veida alerģiskas reakcijas),
gastroenteroloģijā (autoimūns hepatīts, hepatoze, Krona slimība, pankreatīts, aknu ciroze un citi),
ginekoloģijā (smagi noritošs klimakss, premenstruāls sindroms, toksikoze un citi),
kardioloģijā (koronārā sirds slimība, ateroskleroze, miokardīts, autoimūnā kardiomiopātija),
nefroloģijā un uroloģijā (glomerulonefrīts, autoimūnie nefrīti, hronisks prostatīts, un citi ),
endokrinoloģijā (tireotoksikoze, autoimūnais tireoidīts, cukura diabēts, lipīdu vielmaiņas traucējumi un citi),
dermatoloģijā (nātrene, psoriāze, atopisks dermatīts, toksikodermīts, furunkuloze, pemfigus un citi),
saistaudu sistēmas slimību gadījumos (sistēmiskā sarkanā vilkēde, sklerodermija, dermatomiozīts, vaskulīti),
pulmonoloģijā (bronhiālā astma, fibrozējošais alveolīts),
reimatoloģijā (podagra, reimatoīdais artrīts un citi),
neiroloģijā ( migrēna, polineiropātijas, miastēnija, izkaisītā skleroze un citi),
oftalmoloģijā (autoimūns uveīts un citi),
hematoloģijā (Mielomas slimība, krioproteinēmija citi),
ārstnieciskajā kosmetoloģijā (piem.acne un citi),
sporta medicīnā (pienskābes paaugstināta veidošanās, medikamentu un citu vielu lietošana),
toksikoloģijā (alkohola un narkotiskā intoksikācija, medikamentu pārdozēšana),
hroniskās noguršanas sindroma gadījumos.
Aferēzes centrs sniedz ambulatorus pakalpojumus
tiek veiktas plānveida procedūras dienas stacionāra pacientiem un ambulatoriem pacientiem. Tie ir pacienti, kam plazmaferēze nozīmēta, kā papildārstēšana:
autoimūnas saslimšanas – reimatoīdais artrīts, nefrīts, hepatīts, sarkanā vilkēde, hipertireoze,
sistēmas vaskulīti; psoriāze un citas,
pūšļainas dermatozes, pemphigus vulgaris; toksikodermijas,
smagas formas alerģiskas reakcijas;
multipla skleroze.
Ārstnieciskās plazmaferēzes kursu ordinē ārsti ar pieredzi intensīvajā terapijā, anestezioloģijā, neiroloģijā, kardioloģijā, dermatoloģijā u.c. atkarība no pacienta pamata saslimšanas, blakus saslimšanām, vispārējā stāvokļa, klīniskajām un laboratoriskajām atradnēm.
Procedūras norise
Pacients tiek informēts par procedūras iespējamajiem pozitīviem un negatīviem efektiem un paraksta piekrišanu procedūrai.
Procedūras tiek veiktas apstākļos, kur ir iespējams sniegt neatliekamo medicīnisko palīdzību komplikāciju gadījumā. Telpas, kur veic ārstniecisko plazmaferēzi, atrodas stacionārā, kur ir reanimācijas un intensīvas terapijas nodaļa, centralizēta skābekļa padeve, funkcionālas gultas, iespēja veikt kardiomonitorēšanu un defibrilāciju.
Procedūru veic sertificēta transfuzioloģijas māsa sertificēto ārsta-transfuziologa uzraudzībā pēc apstiprinātas tehnoloģijas un darba gaitā ievēro darba drošības instrukciju.
Procedūra tiek veikta ar slēgtu, sterilu, vienreizējas lietošanas maģistrāļu sistēmu komplektu. Pacients plazmaferēzes laikā atrodas gultā, guļus stāvoklī, apsegts, ar mērķi samazināt siltuma zudumu.
Plazmaferēzes procedūra
attīra organismu no vielmaiņas produktiem, liekiem olbaltumiem, taukvielām/holesterīna, toksīniem, antigēniem/antivielām, imūnkompleksiem, medikamentiem,
mazina jebkuras alerģiskas reakcijas (t.sk.bronhiālās astmas lēkmes),
palēnina sarežģījumu progresēšanu cukura diabēta, tireotoksikozes slimniekiem,
palīdz līdzsvarot imūnsistēmu,
mazina niezi un izsitumus ādas problēmu gadījumā,
uzlabo plaušu, nieru un aknu funkcijas,
noņem vai samazina locītavu sāpes,
palīdz samazināt lietojamo medikamentu devas,
uzlabo asinsriti, mazina tūskas un uzlabo audu stāvokli,
palēnina organisma novecošanas procesus.
Plazmaferēzes vēsture īsumā pasaulē
Pirmoreizi Ēģiptē atklāta asins nolaišanas metode (Hipokrāts un Halens);
20.gs. sākumā veikti selektīvas plazmas noņemšanas pirmie mēģinājumi;
1950.gadā Amerikā tika uzsākta asins sadalīšana komponentos.(Ņemceva, 2009)
1970.gadā izgatavoti pirmie automātiskie asins separatori;
1960.-1970.gg. – sākas plašāka plazmaferēzes izmantošana kardioloģijā, neiroloģijā, hematoloģijā, toksikoloģijā;
ASV 1977.g. – 6000 plazmaferēzes procedūru, 1982.g. – 60000 procedūru gadā;
Latvijā
Latvijā kopš 1988.g. pielieto ārstnieciskā plazmaferēzes metodi.
2011.gada 1.augustā tiek atvērts Plazmaferēzes kabinets Rīgas 1. slimnīcā. Pirmo reizi Latvijā parādījās iespēja veikt plazmaferēzes procedūras ambulatori, dienas stacionāra apstākļos, ar iespēju nodrošināt nepieciešamo dienestu klātbūtni.
2015.gadā “Plazmaferēzes kabinets” tiek pārdēvēts par Aferēzes centru. Aprīlī, 2015.gadā ZVA konstatēja, ka Aferēzes centrs ir novērtēts un atbilst Ministru kabineta 2013.gada 22.oktobra noteikumos nr.1176 “Cilvēka audu un šunu izmantošanas kārtība” noteiktajām prasībām un ir tiesīgs veikt šādas audu un šūnu izmantošanas darbības: Mononukleāro šūnu ziedošana, testēšana un ieguve projekta “Randomizēts, dubultakls, daudzcentru, paralēlu grupu III fāzes pētījums DCVAC/PCa efektivitātes un drošības novērtēšanai salīdzinājuma ar placebo vīriešiem ar metestātisku, pret kastrāciju rezistentu prostatas vēzi, kuri piemēroti pirmās līnijas ķīmijterapijai” ietvaros. 2017.gadā – resertifikācija.
Šobdrīd Rīgas 1. slimnīcas Aferēzes centrs ir Latvijā vienīgā ārstnieciskās aferēzes, t.sk. plazmaferēzes, ambulatorā nodaļa.
Citi saistītie pakalpojumi